|
Talking about Photons |
|
|
|
Many popular expositions of physics involving light (or any kind of electromagnetic radiation) refer in a somewhat vague way to “photons”, even though in many cases the explanations would be more aptly expressed in terms of classical electromagnetic waves. Popularizers seem to think that talking in terms of “photons” makes their discussion sound more “modern”, even though their usage of the term is typically very inaccurate, e.g., they often treat “photons” as some kind of localized particles flying through space along definite trajectories, which is not consistent with the concept of photons in quantum electrodynamics. |
|
|
|
To some extent Feynman’s popular book, QED, has misled some casual readers by saying (on page 15) |
|
|
|
I want to emphasize that light comes in this form – particles. It is very important to know that light behaves like particles, especially for those of you who have gone to school, where you were probably told something about light behaving like waves. I’m telling you the way it does behave – like particles. Light is made of particles. |
|
|
|
Notice that he shifts from saying “light behaves like particles” to “light is made of particles”. Later in the same book (page 37) he makes the categorical declaration that “quantum electrodynamics resolves the wave-particle duality by saying that light is made of particles”, even though in the remainder of the book, and in the Feynman lectures, he more accurately explains that “neither the wave viewpoint nor the particle viewpoint is correct”. Indeed, on page 85 of QED he says |
|
|
|
It's rather interesting to note that electrons looked like particles at first, then their wavish character was later discovered. On the other hand, apart from Newton making a mistake and thinking that light was "corpuscular," light looked like waves at first, and its characteristics as a particle were discovered later. In fact, both objects behave somewhat like waves and somewhat like particles. In order to save ourselves from inventing new words such as "wavicles", we have chosen to call these objects "particles," but we all know that they obey these rules for drawing and combining arrows that I have been explaining. It appears that all the "particles" in Nature behave in this quantum mechanical way. |
|
|
|
Thus after his sweeping categorical statements at the beginning, he reverts to the standard view that photons (and electrons, etc.) exhibit both wavelike and particle-like behavior, and when he says we resolve the wave-particle duality by saying light consists of “particles”, he really means we accept the wave-particle duality, and he just arbitrarily chooses to refer to them using the word “particles”, while acknowledging that light does not consist of particles (or corpuscles) in the classical sense of the word. It’s little wonder that some readers have been confused by his comments. |
|
|
|
Strictly speaking, a photon is just an excitation of the quantum field of electromagnetism, and the probability of an exchange of energy by a photon can be described by the wave function or, equivalently (in Feynman’s preferred formalism), by the superposition of the amplitudes along all possible paths (which is the “drawing and combining of arrows” he mentioned above). This accounts for phenomena such as the interference patterns in the two-slit experiment, which can’t be explained by any classical (and local) particles with definite trajectories. |
|
|
|
Another contributor to the popular confusion about photons is the fundamental relation E = hν and the common references to “the frequency of a photon”. Again, strictly speaking, an individual photon interaction cannot exhibit a frequency, because it simply registers as a discrete transfer of energy and momentum. It is true that, in situations such as the two-slit experiment, the probability distribution for the landing point of each individual photon depends on the frequency (of the source), but the landing point of a single photon cannot exhibit that frequency. Many photons must be sent in order to establish the interference pattern that manifests the relevant frequency, which is actually the frequency of the source. Each individual photon emitted by a source characterized by the frequency ν has energy hν, and for an individual photon this energy can (in principle) be measured, but we can’t infer the frequency of the source from that individual photon. |
|
|
|
The fact that every source of electromagnetic radiation (photons) has a frequency should be obvious, but it is often not discussed explicitly in popular expositions. Photons can be produced by many different mechanisms, but the canonical mechanism is simply that of an oscillating electric charge, meaning a charge whose position is cyclically changing, which produces electromagnetic radiation. For example, a single electron oscillating back and forth (e.g., in an antenna or electric motor) produces electromagnetic radiation with a frequency equal to the frequency of oscillation. The energy of each individual photon interaction is proportional to the frequency. Also the phase relations between photons emitted at different times follow the phase of the oscillating charge. For this kind of source it’s easy to understand what “the frequency of the source” means. |
|
|
|
Many readers are also acquainted with the fact that overall neutral atoms can radiate (or absorb) photons when an electron transitions from one energy level to another. In this case it may be less clear what it means to talk about the frequency or phase of such a source. Understanding this requires an understanding of the basic quantum mechanical description of an atom. Recall that early primitive concepts of atoms regarded them as similar to tiny solar systems, with electrons literally orbiting around a nucleus at a certain frequency (revolutions per second), but this presented an inconsistency, because such a situation should classically have resulted in the constant emission of radiation (and hence energy), leading to the electron spiraling into the nucleus. Classically, stable atoms couldn’t exist. |
|
|
|
The quantum mechanical explanation is that the quantum wave function of an electron in the potential well produced by the nucleus has steady-state solutions, in the sense that although the quantum phase is always advancing, the distribution of probability for the location of the electron is stationary (when not excited). Each pair of energy levels acts like an oscillator with a frequency proportional to the difference between those energy levels. Hence the rate of advance of the quantum phase for a transition between those levels is proportional to the energy difference. When an electron changes energy levels, a photon is emitted carrying the quantum phase (which Feynman referred to as the direction of the “arrow”) of the source at the time of emission. The phase of an individual photon does not advance along the null (light-like) path to the destination. |
|
|
|
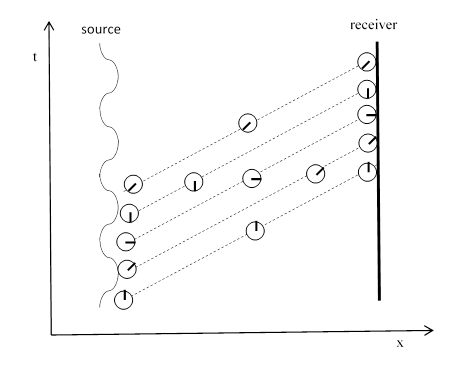
This sometimes confuses people, because that don’t see how light can have a frequency if the individual photons comprising the light don’t somehow oscillate in flight. The explanation is as shown in the figure below. |
|
|
|
|
|
|
|
Each diagonal dashed line represents (crudely speaking) a photon, which carries the phase (represented by the hand on the clock) of the source at the time of emission. At any given time (meaning on any given horizontal slice in the figure) the phase of the light varies from source to receiver, and the light arriving at the receiver has advancing phase as well, but the phase of each photon is constant. A similar drawing is shown as Figure 67 in Feynman’s QED, and his text says |
|
|
|
…the angle of the amplitude for a given path depends on what time the photon is emitted from the source. Once a photon has been emitted there is no further turning of the arrow as a photon goes from one point to another in space-time. |
|
|
|
Unfortunately, Feynman’s figure shows each photon path as a wiggly line, which may mislead some readers into thinking that each photon is somehow oscillating, despite the text making clear that the phase of the photon in transit does not change. In any case, the fact that an individual photon does not oscillate in transit should be obvious, because the elapsed proper time along the dominant light-like path of a photon in a simple context like this is zero, so it wouldn’t even make sense to say that an individual photon oscillates or undergoes any other temporal evolution. |
|
|
|
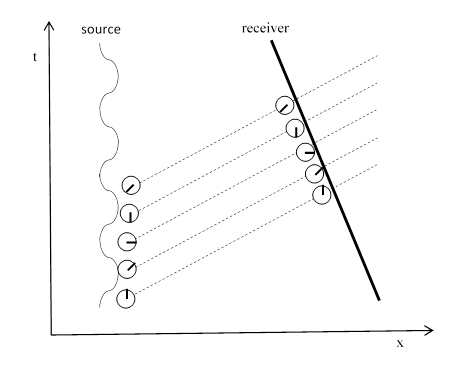
One way of illustrating this is to consider the Doppler effect, which occurs when the distance between the source and receiver is changing. The figure below shows the case when the distance is decreasing. |
|
|
|
|
|
|
|
Since the distance is getting shorter, the receiver encounters the sequence of photons more quickly, so the rate of advancing phase at the receiver is increased. Likewise if the distance is increasing, the receiver encounters the sequence of photons less frequently, so the rate of advancing phase at the receiver is decreased. Notice that if we believed each photon’s phase was changing in transit, and if we regarded the frequency of light as being the rate of change of the individual photon’s phase, then there would be no first-order Doppler effect, because the rate of advancing phase of an individual photon would be the same, regardless of the relative speed. (We refer to first-order, because there would still be second-order Doppler due to relativistic time dilation, but that is negligibly small for typical terrestrial speeds, and it has no directional dependence, because it is proportional to the square of v/c, giving the same factor regardless of whether v is positive or negative.) |
|
|
|
The fact that the Doppler effect applies only to the frequencies of extrinsic variations among a spatio-temporally propagating sequence of entities, not intrinsic frequencies of oscillation of a discrete entity, is one strong demonstration that light is indeed a sequence of entities with different phases, rather than individual entities with evolving phases. To describe this in mundane terms, consider two different cases, one with a single spinning bullet, and one with a sequence of non-spinning bullets each of which is oriented at the angle of the second-hand of a clock (attached to the gun) at the instant of firing. In the first scenario, if the target is approaching the gun, the spin rate of the bullet striking the target is unaffected. This frequency of intrinsic rotation is not subject to the Doppler effect. However, in the second scenario, the rate at which the orientations of the arriving bullets change is subject to the Doppler effect. |
|
|
|
Also, notice that in the second scenario the gun can, in principle, change the intensity of the radiation by increasing the number of bullets per second, without affecting the rate of change of the phase, because all the bullets emitted at a single time carry that phase assuming a coherent monochromatic source. Of course, for any given rate of bullets per second, this frequency is subject to the Doppler effect (as is the overall energy), although it is distinct from the phase frequency. |
|
|
|
As discussed in Elusive Interference, these ideas enable us to account for the fact that a photon (or electron) in a two-slit experiment might be able to land on a certain spot on the screen if just one or the other of the two slits is open, but not if both are open. Consider a light source with a certain frequency and such low intensity that only a single photon (on average) is emitted per hour. Now, quantum mechanics is inherently probabilistic, and we can't predict precisely when the photon will be emitted, nor where it will land. We can only predict the probability distribution for when it will be emitted and where it will land. The probability distribution depends on the available paths for the photon, and also on the frequency of the source. |
|
|
|
The source of the photon, which (again) may be an oscillating charge, or an atom changing energy levels, etc., has a certain "phase", which can be characterized by a little arrow with a certain length and direction, at any given time. The phase of the source is changing with a certain frequency (think of Feynman’s arrow "spinning" like the hand of a clock), and any photon that is emitted from the source carries the phase that the source had at the moment of emission. The phase carried by a photon in transit does not change, but it depends on the precise time when it was emitted from the source. |
|
|
|
Now, since we don't (and can't) know the precise time of emission, nor the precise trajectory, the probability of the photon landing at a particular time and place on the screen of a two-slit experiment is given by adding up the little "arrows" for each possible path, and then the length of the resulting sum for the paths arriving at a given event on the screen is the probability of the photon arriving at that event. From this it’s clear why the interference pattern depends on the frequency of the source, even though the phase of an individual photon along an individual path is constant. At certain locations on the screen, the path lengths via the two slits are different, so the photon would need to have been emitted at different times, with different phases, and those phases may be pointing in opposite directions, so they cancel out (destructive interference), so the probability of a photon landing there is zero, even though it is non-zero if just one of the windows is open. |
|
|
|
|